- At IOS, we are a team of dedicated specialists, nurses, and support staff.
- The Dental office is run by Dr Alberto Barenghi, who is the Health Director, M.D. , D.D.S. and Specialist in Orthodontics since 1992.
- IOS became a Health Dental Care Company in 2012 (Authorization n° 557/05-01-2012 issued by ASL- Lecco), by the acquisition of Alberto Barenghi’s dental office (opened since 1993)
- Our Dental Office is a technologically advanced healthy environment.
- Our goal is to provide clinical services which are characterized by high quality and state of the art facilities.
Profile
- Home
- Profile

Facilities
-
Reception desk:administrative office, company secretary, waiting room , internet access.
-
Dental Clinical Rooms:n° 3 distinct dental clinical rooms, separated, each fully equipped with a dental chair, specific supplies and equipments, digital radiographic system, PC, sink with elbow-operated taps and antimicrobial soap. Suitable indoor air system and lighting. Equipments are treated to routine and planned preventive maintenance. Visibly clean washable surfaces: They are regularly sanitized.
-
Radiology:Separate room with the instrument and accessories for Cone Beam Computerised Tomography and digital scanning .
-
Utility Room for the Decontamination of Equipment: Separate room with suitable indoor air system and lighting, washable surfaces, designated dirty and clean area. It is fully equipped for mechanical cleaning of instruments and autoclave working. It is Regularly sanitized.
-
Holding “Clean” Area:Stockpile in closed drawers inside the dental clinical rooms and in closets placed in the environment rooms
-
Enviroment RoomsFully equipped to support the administrative, clinical and teaching activities. A designated area for clinical waste. Regularly sanitized one.
-
Bathroom:Distinct bathrooms for dental team and patients; equipped with infrared taps and soap dispenser .
-
Aid Station:Ambu device, oxygen tank, defibrillator, monitor for cardiovascular functions, emergency drugs
-
Web Site:Italian and English Version
-
Alberto Barenghi
-
Study and SpecialisationDegree in Medicine(M.D.), Specialist in Dentistry (DDS), Specialist in Orthodontics
-
InterestsHealth Director, orthodontics, paediatric dentistry, dental traumatology, gnathology, oral surgery, fixed and removable prosthetics and , product evaluation, BLSD, radiology.Dental / orthodontics products and equipment evaluation
-
Study and Specialisation
Meet The Team
Guided tour
Digitalising the Dental Office
The choice of computerising all clinical procedures and organizational matters, and of checking the dental office activities is definitive in offering a further assures and efficiency to the patient.
From 1995, the Dental Office has been completely computerised from the clinical point of view (medical records, photographic documentation, radiographic documentation, cephalometric tracings, specialised clinical records, medical prescriptions and Laboratory requests, Sterilisation cycles), and administrative and management documentation (privacy and consent forms, invoicing, etc). In addition, this approach allows a remarkable paper saving.
From January 2012, with the acquisition of the Cone Beam Compurised Tomography device, all the images and 3D reconstructions have been backed-up on the Hardware of the IOS .
The necessity of acquiring and registering breadth of data, has required the construction of a Hardware Network and the interaction of some specific Software. The availability of Patient history is guaranteed by the security in the back-up of the IOS databases.
Ensuring security of the electronic data is a regulation of the Law in regards to management of sensitive data.
We have online documentation in regards to suppliers; the availability of materials from them, up to date stock counts of dental and orthodontic materials, thus allowing for the IOS to complete online orders.
Infrastruttura
-
Reception desk:administrative office, company secretary, waiting room , internet access.
-
Dental Clinical Rooms:n° 3 distinct dental clinical rooms, separated, each fully equipped with a dental chair, specific supplies and equipments, digital radiographic system, PC, sink with elbow-operated taps and antimicrobial soap. Suitable indoor air system and lighting. Equipments are treated to routine and planned preventive maintenance. Visibly clean washable surfaces: They are regularly sanitized.
-
Radiology:Separate room with the instrument and accessories for Cone Beam Computerised Tomography and digital scanning .
-
Utility Room for the Decontamination of Equipment: Separate room with suitable indoor air system and lighting, washable surfaces, designated dirty and clean area. It is fully equipped for mechanical cleaning of instruments and autoclave working. It is Regularly sanitized.
-
Holding “Clean” Area:Stockpile in closed drawers inside the dental clinical rooms and in closets placed in the environment rooms
-
Enviroment RoomsFully equipped to support the administrative, clinical and teaching activities. A designated area for clinical waste. Regularly sanitized one.
-
Bathroom:Distinct bathrooms for dental team and patients; equipped with infrared taps and soap dispenser .
-
Aid Station:Ambu device, oxygen tank, defibrillator, monitor for cardiovascular functions, emergency drugs
-
Web Site:Italian and English Version
How we work
The management of patient and occupational safety is an emerging aspect in Dentistry.
Procedures adopted in the Dental Office:
- The preparation of operating rooms, of instruments and accessories, of staff (suitable hand hygiene, protective apparatus) follow the universal recommendations indicated by the guidelines (CDC 2003, 2008, 2011)
- To safeguard the patients, the staff always use PPE (gloves, masks, glasses, and sterile uniforms) and they use superficial covers (sterile and non, transparent wrap) on high contact clinical surfaces within the operative room.
- We always pack dental devices using paper/plastic tubing cut for instruments in kits, containers or cassettes or singularly (e.g. mirrors, probes, spoons for imprints etc), then sterilized in steam autoclaves e finally conserved in a suitable environment until the use by date (expiry date: 30 days).
- The dental handpieces are also wrapped sterilized.
- All procedures in maintaining the medical equipment used in the practice follow the: a) law b) the guidelines ISPESL-2010 in relation to sterilisation and c) recommendations indicated by 20/SAN/2009 of the Region of Lombardy, harmonised to the specific requirements and definitions in the ‘Procedure of disinfection and maintenance of reusable medical equipment’ (version 1.2013) and the ‘orders of service’ (version 1.2013).
- The following equipments are used during Reusable Medical Equipment Reprocessing:
- Presoaking bath filled with a detergent-disinfectant solution
- Ultrasound baths
- Miele Thermal disinfector G 7881
- 2 autoclaves class B Melag 40
- Water purification system
- Kavo4care got internal intensive cleaning and handpiece equipment lubrication
- Roller Packing Sealer
- Labeller
- Rapid Biological Test Incubator 3M
- Sterilization monitoring (Vacuum test, Helix, Integrator)
- Each piece of dental implants is traceable from manufacturer
- Before every dental care, the high contact surfaces are treated with a rapid action disinfectant and air/surface are periodically disinfected with Nocospray with Evolyse according to the “Procedure of disinfection and maintenance of reusable medical equipment (version 1.2013)”
- Air/surface disinfection with Nocospray with Evolyse is completed before every surgical treatment.
- Periodically, we are subjected to safety walk-around in relation to cleaning, sanitizing, disinfection
- Every dental units water lines is constantly disinfected with ICX according to the manual “Procedure of disinfection and maintenance of reusable medical equipment (version 1.2013)”
- A sterilisable filter cartridge was installed in the STERILAIR DEMA 7 conforming to the guidelines 20/SAN/2009 of Region Lombardy in relation to the rule that ‘the air blown into the mouth must be decontaminated’.
- A verification of each electric medical device and other electric devices for use in a laboratory has been completed according to CEI 61010-1
- A register has been kept on the management and maintenance of equipments.
- Register documenting the period checks of the electric systems, checks of time and operation and efficiency of devices.
- Waste management is disposed by GhibecaTechnoplus srl (PV) according to the law.
- We follow best practice to minimize mercury contamination since each dental chair is equipped with an amalgam separator.
- Qualified expert for estimating the risks of radiation is Dr Antonella Del Vecchio.
- Qualified expert for the Sanitary Supervision of Staff is Dr Giovanni Chiappino.
- Anti-fire course, attended by Dr Alberto Barenghi.
- Update and Training Courses attended by the assistants according to D.Lgs. 81/08
- Our Specialists and Assistants are always upgrading their skills with new instruments, materials and methods.
Patient Safety
- The collection of your health history will be scrupulous.
- It is necessary that you communicate with us any pharmaceuticals that you use, intolerances or allergies to pharmaceuticals or other materials (e.g. metals).
- Prevention: every 6 months we advise a free check-up to our patients.
It verifies the health of the oral cavity and identifies tolerable subclinical situations (e.g. tolerable pain, light gum disease, dental movement) or the initial stage of a tumour, or follows dental changes
Emergency Kit and Defibrillator
Besides the kit and the emergency procedures, a defibrillator and a multiparametric cardiac monitor are present in the dental office; the certification of the DAE operator was obtained (A.A.T 118 Lecco) on the 15/05/2010 by Dr Alberto Barenghi, Dr Livia Barenghi, Ecaterina Cerguta. The operators are required to complete regular course upgrade according to the law.
Clinical procedures of modern Dentistry, Orthodontics, Oral and Orthodontic Surgery, Implantology depend upon the utilisation of protocols, scientifically validated and followed scrupulously (by means of the check list adopted in the hospitals) by using technologically advanced equipment and adequate instruments.
The adoption of such measures is essential in guaranteeing to patients, high-level performance and minimal risks of iatrogenic infection. Such an approach is a safeguard for both the patient and the staff within the dental office.
In safeguarding the patients, both IOS srl and the Professionals within the Dental Office have stipulated a suitable civil responsibility insurance for risks that derive from their professional activity.
References:
- Perea-Perez B. et al. Patient safety in dentistry: dental care risk management plan. Med Oral Patol Oral Cir Bucal. 2011, 16,e805-9
- Henderson SJ. Risk management in clinical practice. Part 11. Oral surgery. British Dental Journal 2011, 210, 17 – 23.
- Thusu S, Panesar S. & R. Bedi R. Patient safety in dentistry – state of play as revealed by a national database of errors. British Dental Journal 2012, 213, E3. doi:10.1038/sj.bdj.2012.669
- Laheij A.M.G.A. et al. Healthcare-associated viral and bacterial infections in dentistry. Journal of Oral Microbiology 2012, 4: 17659, 1-10
- Schaefer MK. et al. Infection Control Assessment of Ambulatory Surgical Centers. JAMA, 2010, 303(22):2273-2279
- SEIEVA 1986-1998. Report ISTISAN 00/32- Board of Health
- Piccinini V e Vulcano F. Sorveglianza delle malattie infettive trasmissibili con la trasfusione (SMITT): ANNO 2006. Notiziario Ist. Sup San 2008, 21, 3
-
We respect all the indications reported in the “Guide for patients in the Dental Office”, edited by the Ministry of Health
An Eco-friendly way in practicing dentistry means that:
- We do not use amalgam for dental restorations to minimize mercury pollution.
- We follow best practice to minimize mercury contamination since each dental chair is equipped with an amalgam separator. Installation of effective separators have shown to eliminate up to 91–99% of the mercury in the waste water from the dental clinic. In the absence of amalgam separator, 33% of Hg in sewers in Canada is due to mercury pollution from dental offices.
- Waste management (including contaminated, hazardous, infectious, regulated medical ones) is disposed according to the Law. We underline that this includes also liquid chemical disinfectants and study casts.
- Digital Radiography avoids the use and waste of chemical developing agents.
- We adopt digital patient records to reduce the amount of the used paper.
- When possible, we use recycled paper.
- We adopt waste management of toner cartridges according to the Law.
- IOS adopts or undertakes to select eco-compatible CE certified dental products
References:
1) Amalgam toxicity—environmental and occupational hazards. Preben Ho¨rsted-Bindslev. Journal of Dentistry (2004) 32, 359–365);
2) Future Use of Materials for Dental Restoration. Report of the Meeting convened at WHO HQ, Geneva, Switzerland 16th to 17th November 2009;
3) Dental Amalgam: Efficacy, Safety and Environmental Issues. Fiona M. Collins. Presentation OSAP symposium 2013
Media
Continuous evolution of dental materials, techniques of intervention, operating protocols, research and technological feasibility are all essential elements for guaranteeing the success of Dental and Orthodontic therapies.
The prudent choice of resistant materials to the dynamic conditions of chemistry and physics (temperature, humidity, acidity, and the presence of microorganisms) occurring in the oral cavity are essential to guaranteeing excellent and stable results in the timeframe according to scientific knowledge.
IOS has searched for trustworthy suppliers with long-standing relationships and reciprocal collaboration, that provides a variety of products of high quality and technological innovation (Kerr Hawe).
IOS has chosen to integrate the expertise of the Prosthetic and Orthodontic Laboratory , and Suppliers of other medical safeguards (e.g. orthodontic vestibular / buccal and lingual Brackets (3M Unitek) , Invisalign, Osseointegrated alloplastic Implants (Nobel Biocare), materials for guided regeneration of tissues, etc).
In realizing the production of prosthesis /orthodontic appliances , IOS has chosen to collaborate with a Prosthetic Laboratory and Orthodontics, in order to utilize the most recent machinery and technology, biocompatible materials and procedures that assure functional excellence and aesthetic durability.
IOS makes use of Prostetic and Orthodontic Lab “ Gilardi & Canella “ to perform customized prosthetic and orthodontic devices. The laboratory “ Gilardi & Canella “, started in Lecco in 1987, and from 1999, its headquarter is placed in C.so Promessi Sposi n° 23/Q. is The registration to Ministry of Health is ITCA01015843. ISO certification has been acquired since 2001.
In its divisions, various prosthetic and orthodontic devices are manufactured:
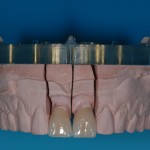
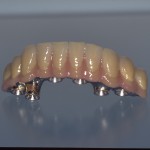
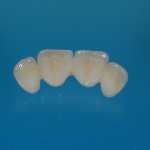
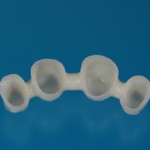
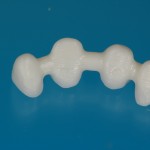
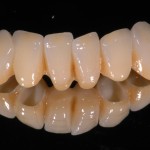
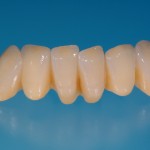
- Fixed prosthesis on teeth and implants (golden alloy ceramic ones, metal free ceramic crows)
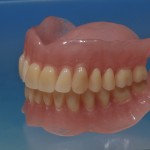
- Removable prosthesis
- Fixed and removable othodontic devices
- Bite-plane for cranio-mandibular disorders
Every customized devices is prepared according the medical specific indication, using CAD – CAM system and innovative materials (zirconium-ceramic and lithium disilicate) to obtain the best performance in terms of functional and esthetic properties.
Only CE certified materials are used. Every prosthetic and orthodontic devices has the certification according to CE directives and National law (Dir 93/42/CEE, D. Lgs 46/97 e 2007/47/CEE, D.Lgs 37/2010), and equipped with instructions.